Why should biomedical engineers care about ocean plastics?
A Q&A with researcher Dr. Bryan D. James
Why should tissue engineers care about the science of ocean plastic? How should biomedical scientists and environmental scientists collaborate? And, most importantly, do I need to drop everything, drive to the ocean, and make as many marine biologist friends as I can find?
Those are just a few of the many questions I had for Dr. Bryan D. James—incoming Assistant Professor in the Department of Chemical Engineering at Northeastern University—when we chatted last month about his research studying the impacts of plastic degradation on both people and planet.
Dr. James leads the EcoBioMaterials Design Lab (which is currently recruiting), dedicated to creating “functional, sustainable, and benign materials for a safer, cleaner future.” He is currently finishing his postdoc at the Woods Hole Oceanographic Institution where he studies the fate, persistence, and toxicity of plastics in the ocean. Before his stint as a Postdoctoral Scholar, James completed his PhD training at the University of Florida in Materials Science and Engineering as an NIH F31 Predoctoral Fellow under the mentorship of Josephine Allen where he worked on a variety of biomaterials and tissue engineering projects, including ones aimed at better understanding how cells respond to biomaterials in a sex-specific manner. He has been a member of the Society For Biomaterials (SFB) national student chapter, previously won the SFB Biomaterials Education Challenge, and is an early career board member of ACS Biomaterials Science & Engineering.
Needless to say, Dr. James changed the way I think about the world, both in and out of the lab.
Most of us are probably used to seeing headlines decrying the state of pollution in our oceans. From the dead seagulls found on beaches with stomachs full of trash to the growth of the Great Pacific Garbage Patch.
News stories like these are what spurred James to start a postdoc studying the many impacts of ocean plastics.
“The ocean has been really near and dear to my heart,” James told me. “We have a lot of sailing background in my family. I was a rower. I’ve always been near or on the water.”
Much of that water, unfortunately, contains plastic, and that ocean plastic continuously fractures into smaller and smaller pieces as it tumbles around the surf in a process called “disintegration.” Disintegration is distinct from “degradation,” during which plastics deteriorate at the molecular level, reducing the molecular weight of the polymer and introducing new functional groups, usually containing oxygen. Degradation can be caused by sunlight (aka photodegradation) and/or microbes (aka biodegradation).
Plastics can undergo both processes simultaneously, but they often disintegrate much faster, sticking around in the form of microplastics as their degradation drags on.
These microplastics have been found almost everywhere. On our beaches. In the food we eat. Even in our organs. A recent paper, published earlier this year, found the plastic polyethylene in 58.4% of patients tested. The patients underwent endarterectomy, a procedure in which plaque is removed from the carotid artery to lower a patient’s risk of having a stroke. This plaque is what the researchers tested for microplastics.
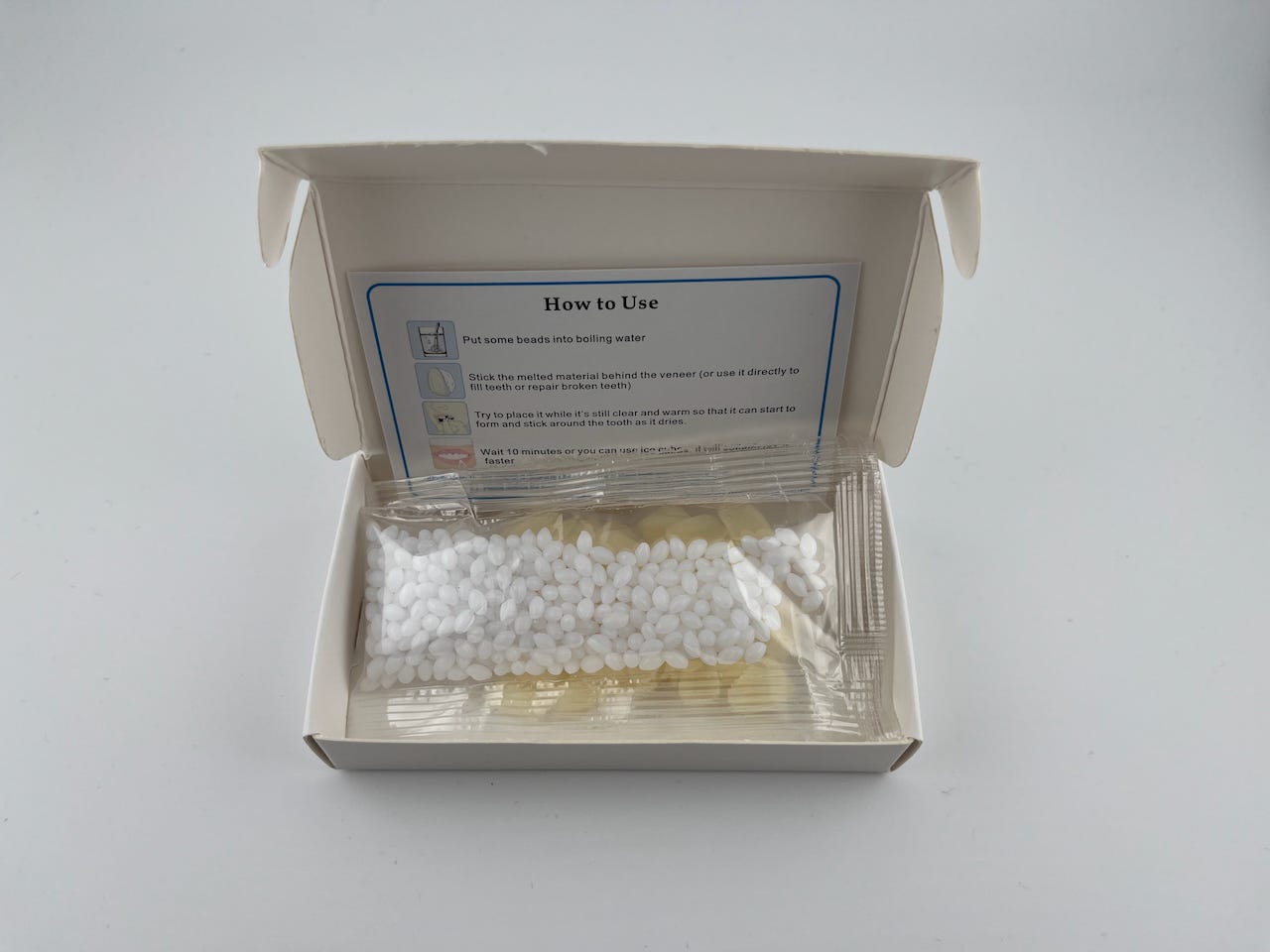
But why should tissue engineers care about how plastics degrade?
Mainly because tissue engineers love plastics! “We use absorbable PLGA or nylon sutures,” the latter which James pointed out is a material often used to make fishing line. “We use polycaprolactone particles, PLGA particles, and liposomes to deliver drugs intentionally into the body.” From scaffolds and stents to drug delivery systems, the majority of regenerative medicine interventions under development involve polymers.
But we should also care about studies of ocean plastics because there’s so much we don’t know about how the degradation of these plastics affects living organisms, both large and small.
“There have been a few studies saying that we found plastic particles in the body,” James said, “suggesting that the presence of microplastics could be associated with increased risk [of developing adverse health conditions], but there's no causative connection yet. The jury is still out on how harmful microplastic or nanoplastic particles are to humans.”
His latest study, published in ACS Biomaterials Science & Engineering last month, helps answer some of those outstanding questions.
“It was a really serendipitous but bittersweet study,” James described, “where we were looking at the developmental toxicity of plastics from the M/V X-Press Pearl ship fire and plastic spill off the coast of Sri Lanka. This [polyethylene] plastic was burned, transformed, full of carcinogenic compounds, much of the same stuff that’s in cigarette smoke, so we were interested to know what the potential risk is for people and wildlife now that that material is floating around the ocean.”
In an unpublished experiment, James and his team incubated zebrafish embryos in freshwater containing these burned polyethylene (PE) pellets. In last month’s study, he also incubated the zebrafish embryos in freshwater containing pellets of thermoplastic polyurethane (TPU) or pellets of polycaprolactone (PCL).
The results surprised him.
While no acute toxicity was observed among the zebrafish embryos when incubated with the polyethylene or thermoplastic polyurethane, the polycaprolactone pellets were acutely toxic. For those concerned about the M/V X-Press Pearl ship fire and polyethylene spill, these results came as a relief, but for those interested in PCL biomaterials, they raised many more questions.
The PCL they tested contained compounds that were found to activate signaling pathways interfering with the metabolism of fats and causing oxidative stress in the embryos, likely contributing to the death of the zebrafish.
It’s important to note that the polycaprolactone pellets James used were sold online as “modeling plastic” and “self-moldable dental replacements” from a variety of sources. “Throughout working on this, I actually broke my front tooth, and I was very tempted to use them to make a false tooth for myself,” James said, an ironic laugh rolling off his tongue.
Unlike biomedical-grade polymers, these consumer-grade materials often have additives that improve their performance, including stabilizers and antioxidants, which James initially believed were responsible for the toxicity.
But James and his team didn’t know exactly which additives were most harmful.
While a few of the additives seemed to be toxic, so too did the PCL itself. When James incubated his zebrafish embryos in freshwater containing a high-purity, biomedical-grade polycaprolactone, more than three quarters of the fish died.
“From that,” James told me, “we concluded that the polycaprolactone oligomers themselves were the likely cause of the toxicity, not necessarily the additives we detected.”
James and the EcoBioMaterials Design Lab will use this data and other datasets like it to create guidelines to help engineers make more informed decisions on what materials to choose for any given application.
James started this effort by understanding how different materials persist or last in the environment. His next goal will be to develop a complementary set of metrics for toxicity.
“If I wanted something that's lightweight and going to last the shortest amount of time in the environment, now we have a metric for doing that. Or if we want it to be the cheapest and also have the shortest lifetime in the environment, you can select a material using this metric. My dream is to develop these types of frameworks for toxicity. For plastics or other chemicals so that you can pair material performance with biological activity, so you don't have to compromise on the usefulness of a design. You can find that middle ground that serves its purpose without causing harm.”
But there’s still a lot of work to be done, especially since every organism may have a different response to any given material.
James gave 6PPD—a chemical used to increase the longevity of rubber tires—as a prime example of this phenomenon. When 6PPD oxidizes, it becomes 6PPD-quinone, which is acutely toxic to Coho salmon. However, researchers have found that “6PPD-quinone isn’t toxic to zebrafish in the same way.”

Importantly, biomedical engineers really like PCL. It’s mechanically strong, making it really well-suited for orthopedic applications, in particular. It takes a few years to degrade in the body, so you don’t have to worry about an implant destabilizing. And it can easily be modified with a variety of useful chemistries. Researchers around the world have put countless PCL implants into both animals and human patients, without noticeable signs of toxicity, but what if there’s more to the story?
If different species can react disparately to the same plastic, could the same be said for the different cell populations in our bodies? Is it possible that PCL, acutely toxic to zebrafish embryos, could cause a cascade of subtle, deleterious changes in human beings?
The FDA published a thorough review of PCL’s safety in 2021, noting that more evidence is needed to characterize the “systemic responses for all PCL device categories.” James agrees, noting there is still important work to be done examining whether or not sub-lethal events occur when different types of cells are exposed to polymeric implants.
“I wouldn't necessarily be worried about acute toxicity [in people]. You’re not going to kill a person or a rabbit or a mouse with PCL. That clearly hasn't happened. But what are the other biological processes that may be affected, not just lethality?
When we evaluate a new biomaterial, the standard practice is to conduct a cell viability assay, such as LDH, MTT, live/dead staining, to prove that this biomaterial isn’t cytotoxic. Then after a functional assay or two, we’ll jump to the animal model and test it. When we’re dealing with an injury, the injury site is already a pretty severe place of damage, so putting in a material may not necessarily reveal a negative impact from an exposure early on.
[Sublethal effects] have become well appreciated in the toxicology space. Consider the history of pesticides. As one class of pesticides were phased out and other persistent pollutants were being found in the environment, researchers discovered that these chemicals acted like hormones. They don't necessarily cause lethality or cancer, but they cause endocrine disrupting effects, which can have substantial, adverse impacts to health, especially during development. As we continue to more broadly employ omic-type techniques, we can monitor a lot of these other potential biological responses that should make for better treatments and a better understanding of how the body responds to materials.”
For James, environmental health and human health are deeply intertwined. You can’t separate one from the other.
In 2022, he published a delightful paper detailing just how valuable collaborations between those two disciplines can be:
“If we’re studying plastics in the environment and we see that a certain plastic particle is accumulating in a specific region of the body, that right there is a really efficient targeting particle for that part of the body. Understanding why that may be happening could inform the design of a better drug delivery platform.
And vice versa, if I’m an environmental scientist is concerned about the release of contaminants from an ingested particle, the models that are used to describe drug delivery behaviors—or in this case the contaminant delivery behavior—have largely been developed in the drug delivery space.”
Perhaps these examples could serve as inspiration for your next research project.




